

Redefining Immuno-Oncology Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Relating to the Amended Registration Statement dated January 9, 2023 Registration File No. 333-265828

This presentation contains forward-looking statements about Genelux Corporation (“Genelux,” “we,” “us” or “our”) that are based on the beliefs and assumptions of our management team, and on information currently available to such management team. These forward-looking statements are subject to numerous risks and uncertainties, many of which are beyond our control. All statements, other than statements of historical fact, contained in this presentation, including statements regarding future events, future financial performance, business strategy and plans, and objectives of ours for future operations, are forward-looking statements. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, which may cause our actual results, levels of activity, performance or achievements of and those of our industry to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on any forward-looking statement. We undertake no obligation to update or revise publicly any of the forward-looking statements after the date hereof to conform the statements to actual results or changed expectations except as required by law. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will” or “would,” or the negative of these terms or other comparable terminology. You should not put undue reliance on any forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved, if at all. Except as required by law, Genelux does not undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise. Trade names, trademarks and service marks of other companies appearing in this presentation are the property of their respective owners. Solely for convenience, the trademarks and tradenames referred to in this presentation appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames. This presentation discusses a product candidate that is under clinical study and which has not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of this product candidate for the use for which it is being studied. Any offering of securities will only be made by means of a registration statement (including a prospectus, filed with the U.S Securities and Exchange Commission (“SEC”), after such registration statement becomes effective. No such registration statement has become effective, as of the date of this presentation. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. We have filed a registration statement (including a prospectus) on Form S-1 (File No. 333-265828) with the SEC for the offering to which this communication relates. Before you invest, you should read the prospectus in that registration statement and other documents we have filed with the SEC for more complete information about Genelux and this offering. You may get these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov. Alternatively, the issuer or any underwriter participating in the offering will arrange to send you the prospectus if you request it by contacting The Benchmark Company, 150 East 58th Street, New York, NY 10155, by email at Prospectus@benchmarkcompany.com or by phone at (212) 312-6700. Forward-Looking Statement

THE POTENTIAL OFFERING 3 Issuer Genelux Corporation Transaction Type Initial Public Offering Securities Offered: 2,500,000 common shares Anticipated Exchange and Ticker Symbol: Nasdaq/GNLX IPO Price Range: $6.00 – $7.00 per share Overallotment Option: 15% Post-Offering Shares Outstanding: 23,647,442 shares Use of Proceeds: Fund the clinical development of our lead product candidate, Olvi-Vec Fund the payment of outstanding accounts payable and accrued liabilities Working capital and general corporate purposes Book Running Manager: The Benchmark Company and Brookline Capital Markets

About Us Genelux is a Phase 3 biopharmaceutical company developing powerful therapeutics for patients suffering from difficult-to-treat cancers. Genelux is focused on the development of next-generation oncolytic viral immunotherapies that are designed to generate a personalized multi-prong attack to overwhelm a tumor’s sophisticated defense mechanisms. Olvi-Vec (olvimulogene nanivacirepvec), is a proprietary, modified strain of the vaccinia virus (VACV), a stable DNA virus with a large engineering capacity having the potential to: Directly kill cancer cells Stimulate a tumor-specific immune response Ability to transform immunologically “cold” tumors into “hot” tumors allowing for responsiveness for immunotherapy Platform technology (ChoiceTM) is the foundation of our oncolytic immunotherapy product development program; and is designed to allow us to generate new product candidates rapidly from conception through the initiation of clinical trials. OUR Lead PRODUCT CANDIDATE OUR SCIENCE

Seasoned Leaders with Extensive Business & Clinical Development Experience Thomas Zindrick, JD – Chair, President & CEO 30+ years industry experience (Former – Amgen, VP) The Genelux Team Tony Yu, PhD – Head of Development 20+ years industry experience Qian Zhang, MD, PhD – Assoc. VP, Research 15+ years industry experience Ralph Smalling, MS – Head of Regulatory Affairs 35+ years industry experience Joseph Cappello, PhD – Head of Operations 30+ years industry experience Caroline Jewett – Head of Quality 30+ years industry experience Robert Holloway, MD – Chairman Robert L. Coleman, MD Alberto A. Mendivil, MD Paul Scigalla, MD, PhD – Chief Medical Officer 35+ years industry experience (Former - Pfizer, VP; Boehringer Mannheim, SVP) Thomas J. Herzog, MD David M. O’Malley, MD Clinical Advisory Board Executive Leadership Operations James L. Tyree, MBA – Lead Independent Director 35+ years industry experience (Former - Abbott Global Pharmaceuticals, EVP) Doug Samuelson – Chief Financial Officer 30+ years industry experience (Former – Wellness Center USA, CFO) Sean Ryder, JD– General Counsel 20+ years industry experience (Former – Helsinn Therapeutics (US), VP) R&D

Investment Thesis Broad Technology Platform Advanced Clinical Program Identified Commercial Strategy Large Market Opportunity Phase 3 registration trial actively recruiting patients (late-stage ovarian cancer) Proof of Concept confirmed in Phase 2 trial Phase 2 trial actively being prepared for initiation (recurrent non-small cell lung cancer; i.v. route) Dose-dependent survival benefit in Phase 1b monotherapy study Potential utility against broad range of tumor types and metastatic disease Physician-preferred/familiar route(s) of administration, e.g., intravenous delivery 500+ novel strains generated via our proprietary CHOICE™ platform Five-year US sales forecast estimated at $1B+ (post-marketing approval of Olvi-Vec) Potential in multiple clinical settings offer significant revenue upside US launch in ovarian cancer; strategic partnership for larger indications Exclusive licenses outside the US (Newsoara Collaboration Agreement established in 2021) Validating Strategic Partnerships Newsoara BioPharma Co. Ltd. (Chinese rights) anticipates initiating 3 Phase 1/2 clinical trials with Olvi-Vec ELIAS Animal Health (Worldwide rights) anticipates initiating canine efficacy studies with V-VET1

Pipeline Believed to be the most-advanced, non-local delivery Oncolytic Virus clinical program. U.S. Revenue Projection (5-yr post-marketing approval) Ovarian: $250M Total: $1B+ Additional Revenue Opportunities Re-treatment Front-line cancer Additional Indications $1B+ 1 Commercial Rights 1aGenelux: Worldwide (excluding Greater China); Newsoara (Greater China) 1bV2ACT Immunotherapy: Worldwide (excluding Greater China) 1cELIAS: Worldwide 2 We have enrolled our first patient in our Phase 3 clinical trial. 3 Based on the results of our previously completed Phase 1 clinical trials of Olvi-Vec administered intravenously to patients with solid tumors, we are planning to initiate a Phase 2 clinical trial of Olvi-Vec in recurrent NSCLC. 4 Newsoara has submitted an IND and protocols to the Chinese National Medical Products Administration 5 V2ACT has an active IND for this product candidate. The Phase 1b/2a clinical trial is not yet scheduled to be initiated. 6 ELIAS is developing an efficacy trial.

Genelux is focused on building a fully-integrated therapeutics company. Late-Stage Clinical Program Initiated Phase 3 registration trial in late-stage Ovarian cancer Initiate Phase 2 trial in recurrent Non-Small-Cell Lung cancer Strategic Partnerships Newsoara anticipates initiating 3 China-based Phase 1/2 clinical trials ELIAS anticipates initiating canine efficacy study(ies) In-house cGMP Manufacturing Facility Build-out of in-house production facility in San Diego, CA Produce additional GMP batches to meet supply requirements Near-Term Milestones Advanced Clinical Program Broad Technology Platform Identified Commercial Strategy Validating Strategic Partnerships Large Market Opportunity


Our Lead Product

Olvi-Vec (olvimulogene nanivacirepvec) Physician-preferred methods of delivery locate and kill cancer cells to enhance antigen presentation and stimulate an anticancer immune response Immunostimulatory backbone, by turning the tumor “hot”, for combination therapy with other therapies, including chemotherapies Patients who received Olvi-Vec-primed immunochemotherapy may respond to chemotherapy to which they previously were deemed resistant or refractory Addressing significant unmet medical needs. A differentiated, and desirable immuno-oncology approach Signals of Differentiated Therapeutic Potential Oncolytic Vaccinia (Olvi-Vec) Primed Immunochemotherapy

No third-party royalties due 40 patents issued Composition of matter / use United States expiry 2031 (includes PTE) Manufacturing United States expiry 2038 Marketing exclusivity biosimilar entry United States: 12 years European Union: 10+1 years/CA & JP 8 years PRC (China): (pending 6-12 years) Intellectual Property: Freedom to Operate and Market Exclusivity Genelux technology protected as Trade Secrets Worldwide Operating Freedom Extensive Patent Estate Proprietary Manufacturing IP “Moat” Long-Duration Regulatory Exclusivity

Clinical Trial Results

High & Condensed Dosing All patients received a single cycle of Olvi-Vec Bolus infusions (intraperitoneal delivery) on 2 consecutive days, i.e., total dose: 6x109 pfu Heavily pre-treated with documented progressive disease at baseline No Standard of Care i.e., clinical trial or palliative care Platinum-resistant / refractory Patients Phase 1b: Olvi-Vec Monotherapy (11 patients) Tolerability: Toxicity: No Dose Limiting Toxicity (DLT) No Maximum Tolerated Dose (MTD) Most Common Adverse Events (AE): Transient, flu-like symptoms Abdominal pain (Grades 1 & 2) No Grade 4 AEs Antitumor activity: Clinical Benefit Rate: 73% (8/11) 4/11 patients had >2x PFS relative to immediate prior chemotherapy Translational Evidence: Activation of tumor-specific T cell response detected in blood Documented immune activation in tumor microenvironment with significant influx of tumor infiltration lymphocytes Favorable immune-related genetic signatures (via biomarkers) Ovarian Cancer Program: VIRO-15 Phase 1b/2 trial Manyam M, et al, Gynecologic Oncology 163 (2021) 481–489 Demonstrated anti-tumor activity as monotherapy and combination therapy

Ovarian Cancer Program: Platinum-resistant / refractory Ovarian Cancer Oncolytic Vaccinia (Olvi-Vec) Primed Immunochemotherapy in Platinum-Resistant / Refractory Ovarian Cancer Olvi-Vec Platinum-based doublet +/- Bev Olvi-Vec- Primed Immuno- chemotherapy Preestablished endpoints met Robert W. Holloway, et al 54% RECIST response (vs.<15-20% historical to chemotherapy) 11.0 mo. of median progression-free survival (PFS) (vs. ~5 mo. historical) Platinum-refractory (13 patients) 54% RECIST response (vs. <10% historical); 7% response to VIRO-15 patients’ most recent prior platinum line 11.4 mo. of median progression-free survival (PFS) (vs. ~3 mo. historical) 1Platinum doublet +/- Bevacizumab 2VIRO-15 patients had results in prior lines of therapy similar to historical data. 3RECIST readings based on pre-chemo baseline. Phase 2: Olvi-Vec followed by chemotherapy1 (Clinical Benefit2&3) All PRROC (27 patients)

Olvi-Vec-Primed Immunochemotherapy 36 yrs old Stage IIIB, papillary serous ECOG: 0 BRCA negative MSI: stable # of mutations (load): 4 (low) PD-L1 negative BOR: PR by CA-125 & CT scan Overall survival: 23.2 months 15B-01: 15B-17: Patient who progressed while on last platinum, presented at time of enrollment with progressive disease and projected short life expectancy. All achieved PFS exceeding any of their respective prior lines, and achieved objective partial response, indicating meaningful clinical benefit from Olvi-Vec-primed immunochemotherapy. 65 yrs old Stage IIIC, high grade serous ECOG: 1 BRCA negative BOR: PR by CA-125 & CT scan Overall survival: 15.7 months 15B-15: 67 yrs old Stage IIIB, high grade serous ECOG: 0 BRCA negative # of mutations (load): 0 (low) PD-L1 negative BOR: PR by CA-125 & CT scan Overall survival: 12.3 months Exemplary heavily pre-treated platinum-refractory

Rapid, Common and Durable Responses platinum-resistant platinum-refractory 4 patients achieved 100% reduction of target lesions (even in a platinum-refractory patient with heavy tumor burden) All PRROC Patients: 86% Platinum-refractory patients: 91% Tumor Shrinkage All PRROC Patients: 7.6 months Platinum-refractory patients: 8.0 months Duration of Response Change of target lesion size (% change of SLD) Best change of target lesions from baseline (%) Anti-tumor Activity: Tumor Shrinkage

Clinical benefit: Long-term Overall Survival Benefit flat tail Demonstrated Survival Tail (~20%), a hallmark of Clinically Beneficial Immunotherapies Durable Benefit: ~ 20% long-term survivors (median follow-up time: 44.5 mos) VIRO-15: Kaplan-Meier OS Curve Historical Data 2nd line 21.1 mOS (95% CI, 17.8–24.4) 3rd line 12.0 mOS (95% CI, 10.4–14.6) 4th line 9.8 mOS (95% CI, 7.1– 12.25) 5th line Not reported [Bookman et al., Gynecol Oncol. 2017;146(1):58-63] VIRO-15 5th line (med) 15.7 mOS (95%, 12.3 – 23.8)

Competitive Landscape

VIRO-15 Phase 2 Results: Comparison with Seminal Trials in Ovarian Cancer Currently 230,000+ ovarian cancer patients in the United States While clinical remissions are obtainable, a majority of patients will relapse (~80%) 54% 85% 77% 0% 20% 40% 60% 80% 100% RECIST1.1 CA-125 PFS-6-month Study # or prior lines Regimen Platinum-resistant / refractory patients AURELIA1 ≤ 2 prior lines Chemo + Avastin (i.e., CT+Bev) B-GEMOX2 1-2 (21%), 3-4 (63%), ≥ 5 (16%) Oxali + Gem + Avastin FORWARD II3 Median 3 prior lines Mirvetuximab soravtansine + pembrolizumab (median/high FRα group) VB-1114 ≤ 3 prior lines VB-111 + paclitaxel JAVELIN-200 ≤ 3 prior lines Avelumab + PLD TOPACIO5 ≤ 4 prior lines Niraparib + pembrolizumab VIRO-15 Median 4 prior lines Olvi-Vec / Chemo ± Avastin Platinum-sensitive patients OCEANS6 No prior chemo in recurrent setting Carbo + Gem + Avastin Driver of Market Penetration 20 References (1)Pujade-Lauraine et al., J Clin Oncol 2014;32:1302-1308. (2)Ikeda et al., Int J Gynecol Cancer 2013;23:355-360. (3)Matulonis et al., ESMO 2018. (4)Arend et al., Gynecol Oncol. 2020;157:578-584. (5)Konstantinopoulos et al., J Clin Oncol 2018;36(S15)106. (6)Aghajanian et al., Gynecol Oncol. 2015;139(1):10–16. Footnote: As the data presented is based on a cross-trial comparison and not a head-to-head clinical trial, such comparisons may not be reliable due to differences in study protocols, conditions and patient populations. Accordingly, cross-trial comparisons may not be reliable predictors of the relative efficacy or other characteristics of our candidates compared to others presented.

Systemic Program: Dose-dependent survival benefit of Heavily Pre-treated Patients Dose Escalation Phase 1b Monotherapy Study in Solid Tumors Progressed from Last Prior Therapy Median 5 prior lines of therapy Regimen: various dosing levels and schedules (typically over 4-6 months) Well tolerated: no DLT or MTD reached Clinical Benefit: statistically significant in primary / metastatic lung diseases | Data below Virus dose is in Total Cumulative Dose (TCD) received in all cycles in each patient Demonstrated feasibility of multiple IV cycles

Systemic Program: Condensed Dosing followed by Chemotherapy Case Report (Pt #21A-06) SLD of nontarget lesions (mm) Recurrent metastatic cervical cancer with lung mets Received 5 consecutive daily i.v. doses Transient adverse reactions: fever, nausea, bone pain (Hx arthritis) Stable disease with no tumor size increase Phase 1 Study High and Condensed Dosing (Single round: bolus infusion on 5 consecutive days) Summary Well tolerated, with No DLT or MTD reached. Anti-tumor effects of monotherapy treatment Virus treatment revitalizes tumors to subsequent chemotherapy Demonstrated Anti-tumor effect of IV immunochemotherapy High-grade pancreatic cancer with lung & liver mets Received 5 consecutive daily i.v. doses Transient adverse reactions: fever, nausea 59% drop of CA19.9 tumor biomarker and Objective Response per RECIST, with PFS of 18 weeks Case Report (Pt.#21A-04) Best change (%) of target lesion sizes from baseline by RECIST Partial response (NCT03420430/FHCI) Chemotherapy after disease progression Partial Response PFS: 70+ wks OS: 46.4 mos (ongoing) Chemotherapy after disease progression 83% drop of CA 19.9 Partial Response by RECIST PFS: 31 wks

CHOICE™ Discovery Platform

Viral vectors selected based on multiple in vitro and in vivo selection criteria Extensive library of viral vectors with a variety of anti-tumor attributes Regression and elimination of a wide range (20+) of tumor types in pre-clinical models Comprehensive Approach Highly Productive Broad Utility CHOICE™ discovery platform Flexible, powerful and modular
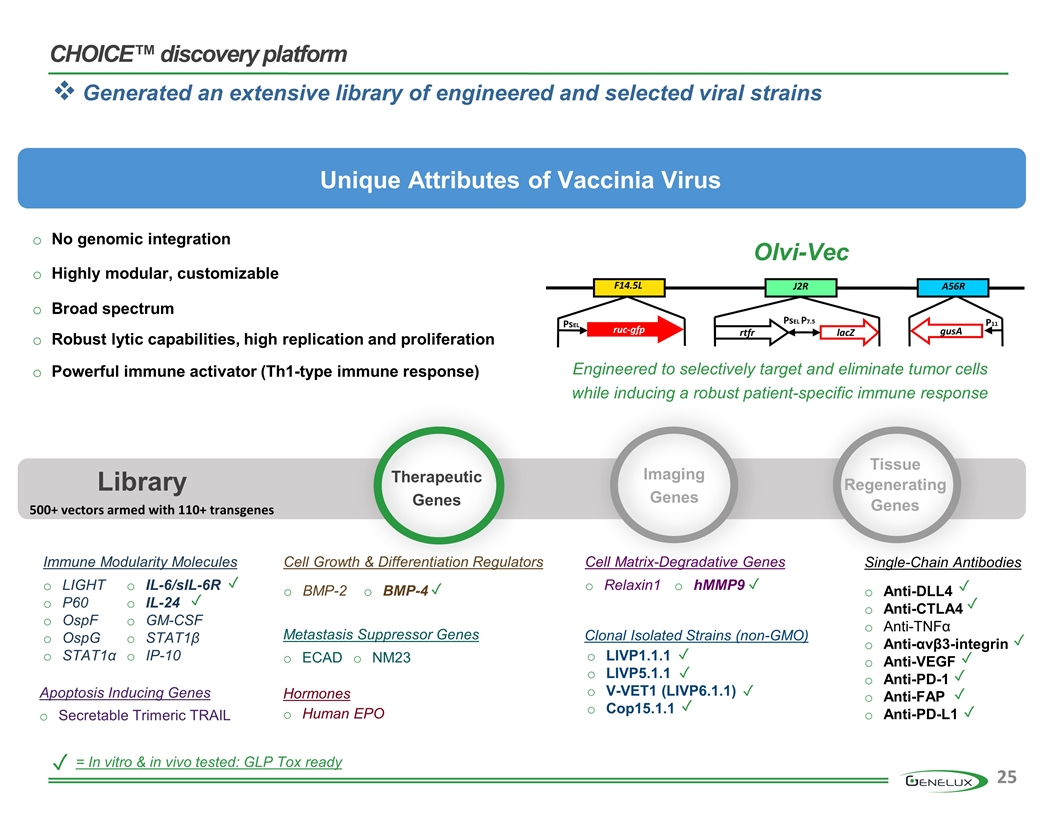
CHOICE™ discovery platform Unique Attributes of Vaccinia Virus No genomic integration Robust lytic capabilities, high replication and proliferation Highly modular, customizable Broad spectrum Powerful immune activator (Th1-type immune response) = In vitro & in vivo tested: GLP Tox ready ✓ Library Therapeutic Genes Imaging Genes Tissue Regenerating Genes PsEL P7.5 lacZ ruc-gfp PsEL gusA rtfr J2R F14.5L A56R P11 Olvi-Vec Immune Modularity Molecules LIGHT P60 OspF OspG STAT1α IL-6/sIL-6R IL-24 GM-CSF STAT1β IP-10 Metastasis Suppressor Genes ECAD NM23 Cell Matrix-Degradative Genes Relaxin1 hMMP9 Hormones Human EPO Cell Growth & Differentiation Regulators BMP-2 BMP-4 Apoptosis Inducing Genes Secretable Trimeric TRAIL ✓ ✓ ✓ ✓ LIVP1.1.1 LIVP5.1.1 V-VET1 (LIVP6.1.1) Cop15.1.1 ✓ ✓ ✓ ✓ Clonal Isolated Strains (non-GMO) Single-Chain Antibodies Anti-DLL4 Anti-CTLA4 Anti-TNFα Anti-αvβ3-integrin Anti-VEGF Anti-PD-1 Anti-FAP Anti-PD-L1 ✓ ✓ ✓ ✓ ✓ ✓ ✓ Generated an extensive library of engineered and selected viral strains Engineered to selectively target and eliminate tumor cells while inducing a robust patient-specific immune response 500+ vectors armed with 110+ transgenes

Facilities and Operations

Facilities and Operations: Based in Southern California Established and equipped an independent, Company-controlled 7,500+ Sq. Ft manufacturing facility in San Diego to secure material for pivotal studies and potential commercial supply Genelux maintains agreements with raw material and equipment suppliers, as well as contract labs to provide supply chain redundancies and flexibility to offload certain services to CMOs / CROs Genelux maintains agreements with third-party companies for labeling, packaging, distribution of both clinical material as well as future potential commercial products Genelux plans to invest in and augment its internal development capabilities as well as continually improve its proprietary manufacturing processes Integrated R&D and manufacturing capabilities Genelux has developed a large-scale cGMP manufacturing process to optimize production Headquarters Westlake Village, CA, USA Research and Development San Diego, CA, USA Manufacturing Facility San Diego, CA, USA

Cap Table Summary Share Count Note Common Stock 21,147,442 (1) Common Stock – IPO 2,500,000 Excluding Overallotment Basic Shares Outstanding Post-IPO 23,647,442 Plus: Other Issued Dilutive Instruments Stock Options Outstanding 4,201,018 Warrants Outstanding 734,841 Issuable upon the optional conversion of certain convertible promissory notes 5,461 Warrants to be issued upon conversion of convertible debt 183,852 5,125,172 (2) Fully Diluted Share Count 28,772,614 Anticipated Use of Proceeds: The company intend to use the net proceeds from this offering to fund the clinical development of our lead product candidate, Olvi-Vec; to fund the payment of outstanding accounts payable and accrued liabilities; and for working capital and general corporate purposes The number of shares of our common stock to be outstanding after this offering is based on 21,069,841 shares of common stock outstanding as December 31, 2022, after giving effect to (i) the automatic conversion of certain convertible promissory notes and accrued and unpaid interest and loan fees thereunder as of December 31, 2022 into 3,394,569 shares of common stock; (ii) the automatic conversion of all outstanding shares of our convertible preferred stock into 8,355,610 shares of common stock; and (iii) the issuance of 270,537 shares of common stock upon satisfaction of earned and unpaid dividends on our Series H preferred stock as of December 31, 2022, each in connection with the closing of this offering. The number of shares of our common stock to be outstanding after this offering excludes 2,800,00 shares of our common stock reserved for future issuance under our 2022 Equity Incentive Plan and 700,000 shares of our common stock reserved for issuance under our 2022 Employee Stock Purchase Plan, which will both become effective once the registration statement is declared effective


platinum-resistant platinum-refractory Baseline CA-125 value (U/mL) All 11 patients with > 90% decrease of CA-125 achieved RECIST response RECIST responses correlate to CA-125 responses (p = 0.007) All PRROC Patients: 96% (25/26) Platinum-refractory patients: 92% (12/13) CA-125 Decrease All PRROC Patients: 85% (22/26) Platinum-refractory patients: 85% (11/13) ORR by CA-125 Rapid, Common and Durable Responses Anti-tumor Activity: CA-125 Biomarker

Meaningful clinical benefit: Relative to Patients’ Immediately Preceding Line of Therapy (Von Hoff et al., J Clin Oncol. 2010;28(33):4877-83) Historically, the proportion of patients achieving a response and duration of response decreases with each subsequent line of therapy ‘PFS ratio’ [= (PFS+1 on investigational treatment) / (PFS-1 on last prior therapy)] PFS+1 post-Olvi-Vec mPFS: 11.0 mos mPFS 4.5 mos PFS-1 pre-Olvi-Vec (last prior line) 1 [PFS-6 mos: 29%] Using Patients as own control [PFS-6 mos: 77%] 1VIRO-15 patients had results in prior lines of therapy similar to historical data

Olvi-Vec Gene expression analysis (NanoString RNA profiling) Chemotherapy + Avastin Immunogenic cell death Vascular collapse Immune activation Increased TILs & memory T cells Inhibition of anti-viral response Immunogenic cell death Depletion of suppressor cells Increase susceptibility to CTLs Positive regulation of T-cell activating and trafficking - up-regulation: IGF2R, DPP4, STAT1, TRAT1, VCAM1 Inhibition of anti-virus response - down-regulation: IFI6, IFITM1, MX1, OAS3 Promotion of sensitization/response to chemotherapy - up-regulation: IGF2R, STAT1 - down-regulation: BRD3, DUSP1, IFI6 Correlation of better prognosis in cancer patients - up-regulation: IGF2R, STAT1, TRAT1 - down-regulation: BRD3, DUSP1, IFI6, MX1, EIF2AK2 Modulating the Tumor Microenvironment: Overcoming Chemoresistance Complementary mechanisms of Olvi-Vec-primed Immunochemotherapy

Activation of Immunosurveillance baseline Day 5 Olvi-Vec Treatment on Days 3 & 4 Day 10 Clusters of tumor cells Limited immune cells Drastic reduced tumor cells Large increase of immune cells Oncolysis Immune activation (VIRO-15: monotherapy) peritoneal fluid Prior to 1st dose W2D17 W2D12 pleural fluid W2D9 W2D15 W2D17 Robust lymphocyte count increase Elimination of Cancer Cells after Increased Lymphocyte Infiltration in different cavities Tumor cell clusters present Tumor cell clusters absent (Pt.#15A-11) (Pt.#15A-01) Mechanism of Action: Oncolysis & Immune Activation

Pre-Olvi-Vec Post-Olvi-Vec (prior to subsequent chemotherapy) Pt# 15A-16 CD8+ CD4+ Pt# 15A-18 CD8+ CD4+ Pt# 15A-23 CD8+ T-cells: Infiltrating Lymphocytes are Prognostic for Response and Survival Induced Infiltration of CD8+ cells into Tumors Endogenous TILs (intra-tumoral and stromal) are very low in ovarian cancer Shift of CD8+ cells into epithelial tissue 42 14 705 146 126 45 494 109 107 69 135 28 Baseline Screening Post – Treatment Pt# 15A-18 Pt# 15A-23 Pt# 15A-16 Outside Tumor Inside Tumor Outside Tumor Inside Tumor Outside Tumor Inside Tumor Pt#

Off-the-Shelf Personalized Medicine: Single Agent generates Individualized Results Long-lasting, Tumor-specific T cell response corresponds to tumor reduction Heavily pre-treated 9 prior regimens of chemo+Avastin; no pre-existing tumor-specific T-cells Post treatment Consequential amount (~3%) of all activatable T cells at Week 30 are tumor-specific T-cells Case Report (Pt #15A-05) No TSTcR 0 18 36 -24 Last Chemotherapy: Pemetrexed 3/30-5/18/2016 Progressive disease (PD) (CT scans on 6/30/2016 & 8/30/2016) GL-ONC1 Tx Partial response (confirmed on Week 24 & 36; durable) Stable disease @ Week 48 (-27% compared to baseline) Confirmed TSTcR Confirms tumor-antigen presentation & immune activation/memory (VIRO-15: monotherapy)

Ovarian Cancer Program: Phase 3 registrational-stage clinical trial design in PRROC patients Olvi-Vec Clinical program: Olvi-Vec-primed Immunochemotherapy trials Progression on last platinum: 0-6 mos Time from last platinum: 3-15 mos Patient Population: N=186 No standard of care therapy Number of prior lines of therapy: ≥3 Progression-free survival (RECIST1.1) Primary Endpoint Olvi-Vec IP Qdx2 (week 0) Platinum doublet + bevacizumab (week 4) (Single-agent non-platinum + bevacizumab maintenance) Platinum doublet + bevacizumab (week 0) (Single-agent non-platinum + bevacizumab maintenance) R A N D O M I Z E Experimental Arm Active Comparator Arm W/ successful catheter implantation W/o successful catheter implantation 2:1 Sponsor Trial Sites Indication Clinical Stage Patients (est.) Randomization US Recurrent NSCLC Phase II ~138 2:1 China Recurrent OC Phase I/II ~150 2:1 China Recurrent NSCLC Phase I/II ~150 2:1 China Recurrent SCLC Phase I/II ~150 Single arm Systemic Program: Clinical Trials